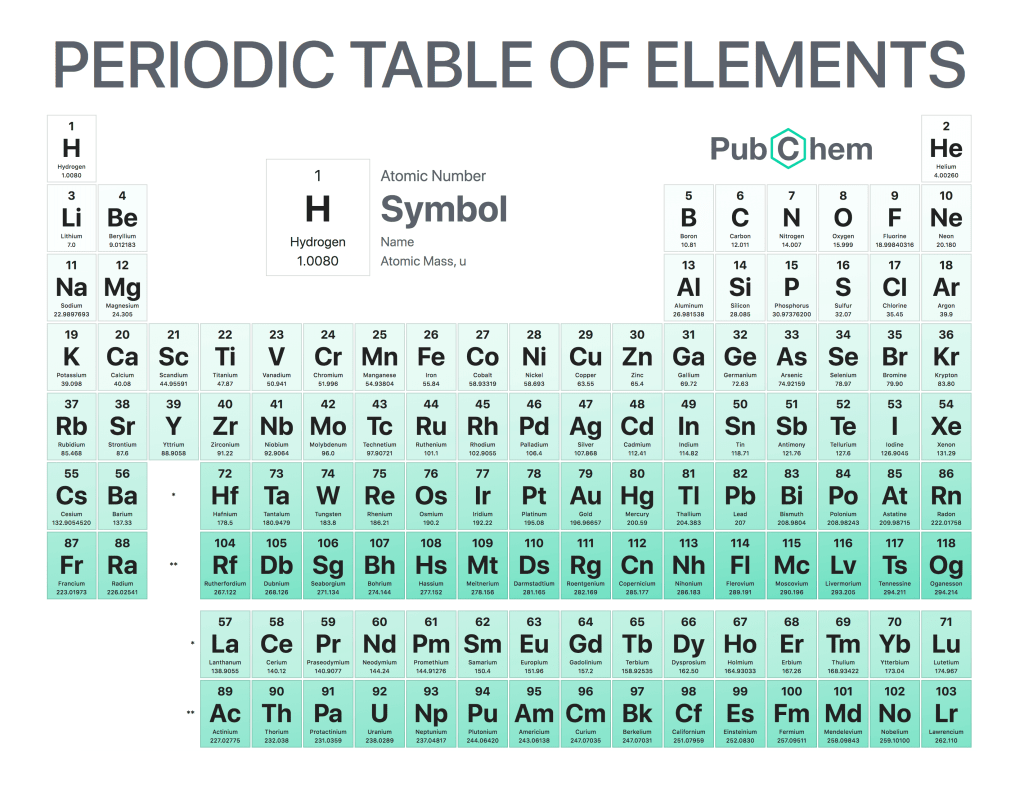
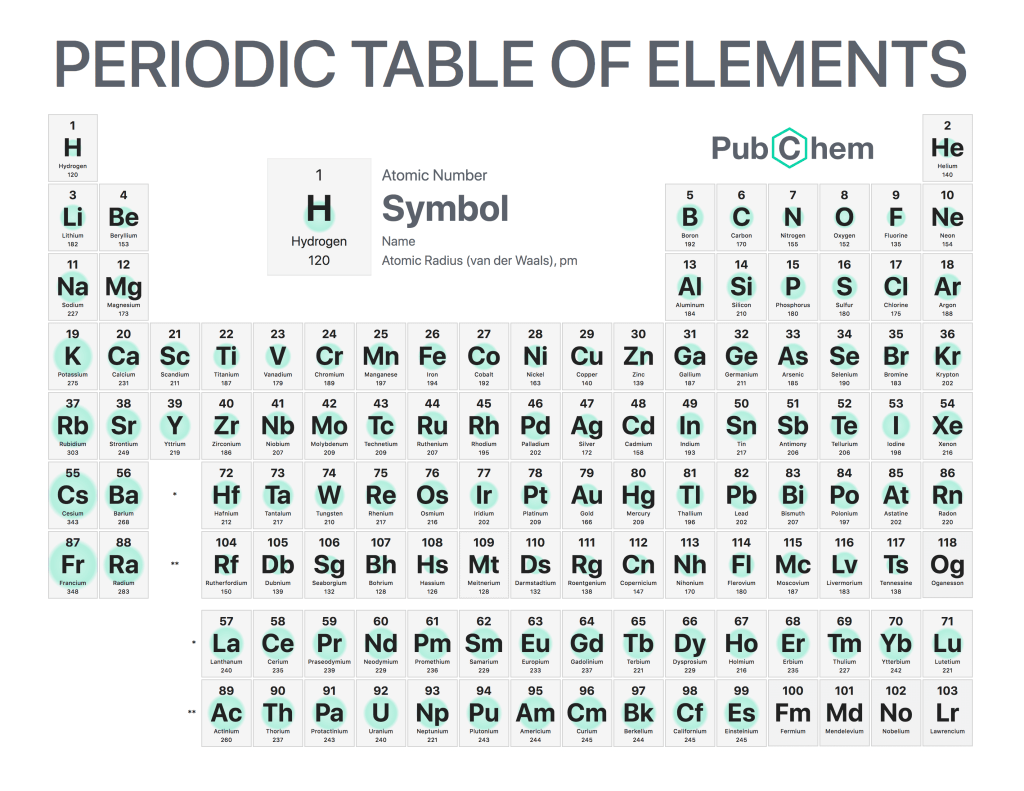
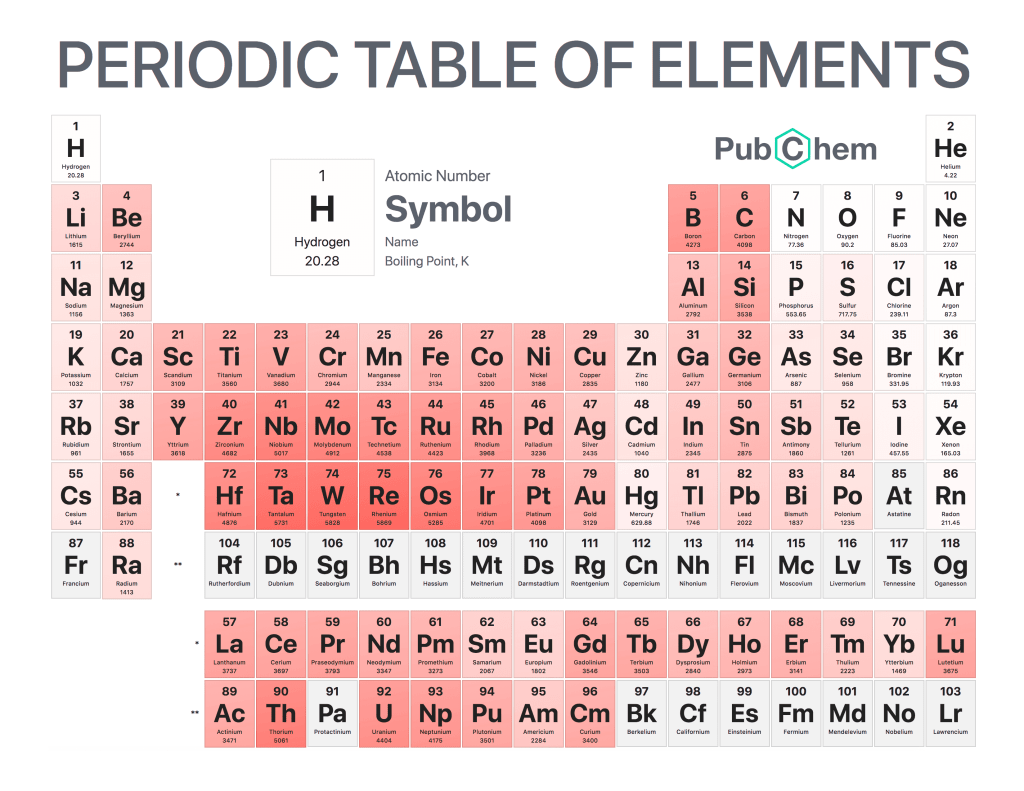
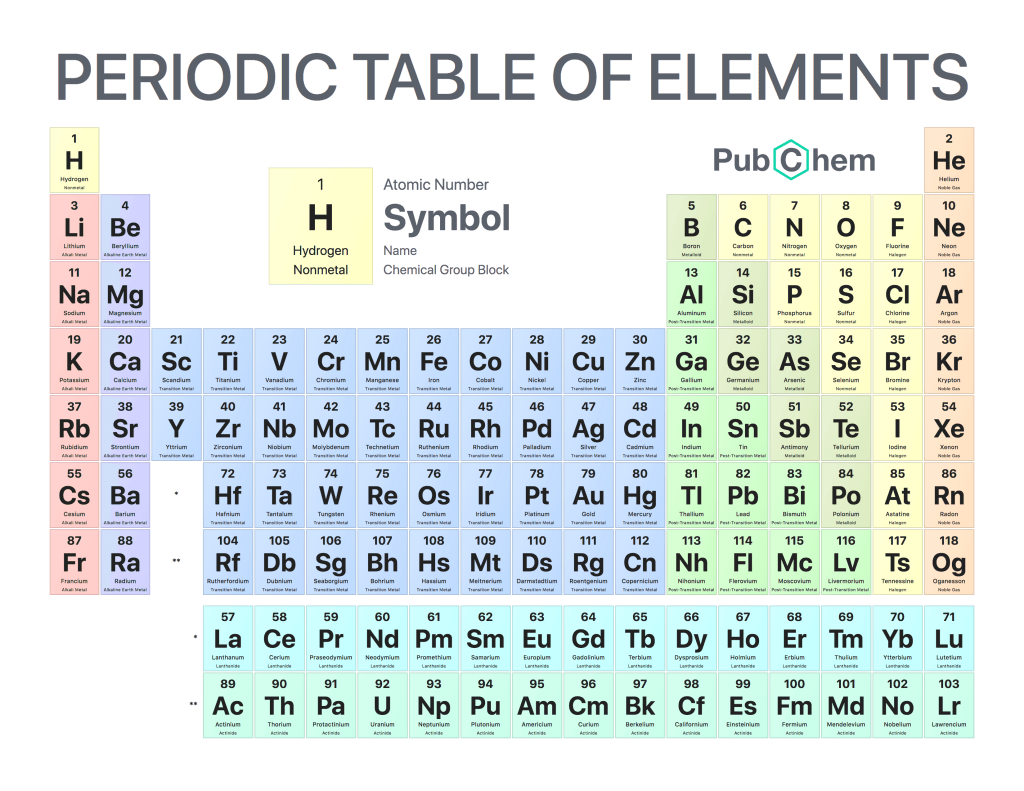
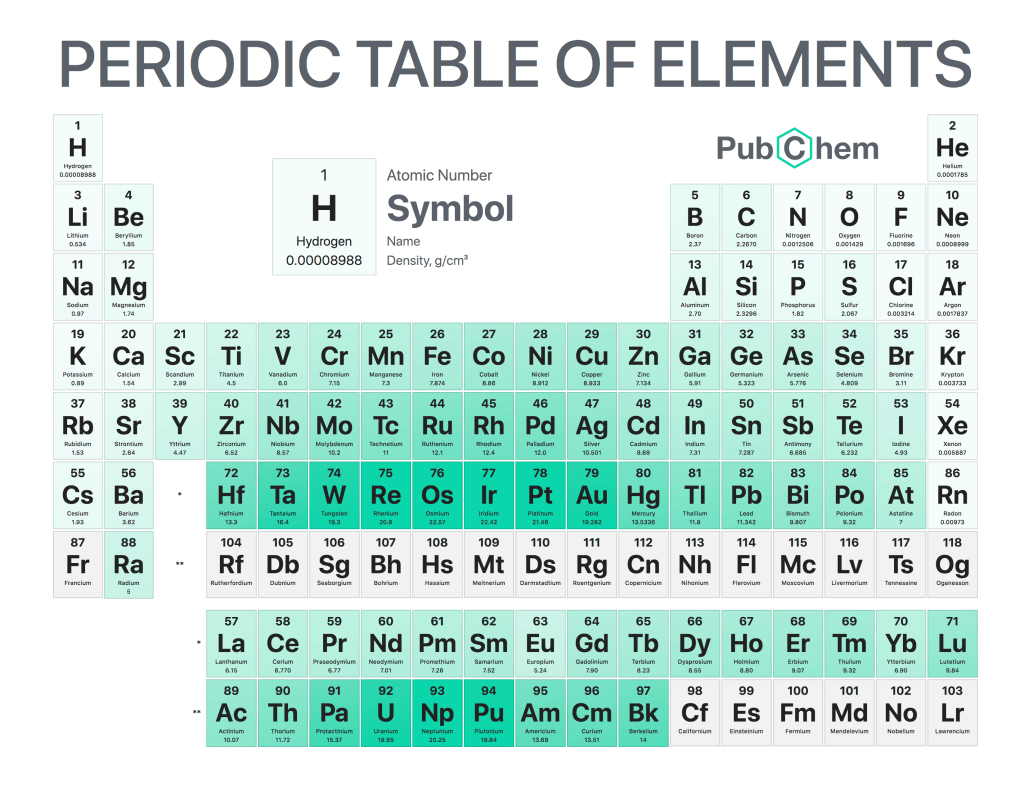
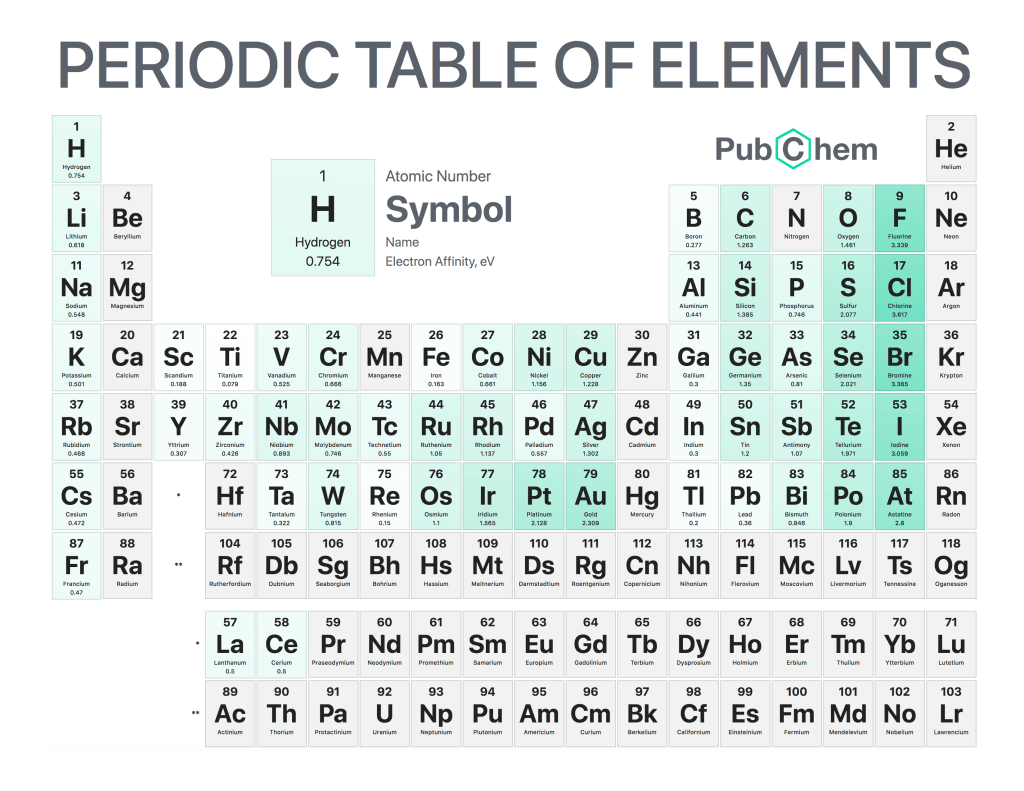
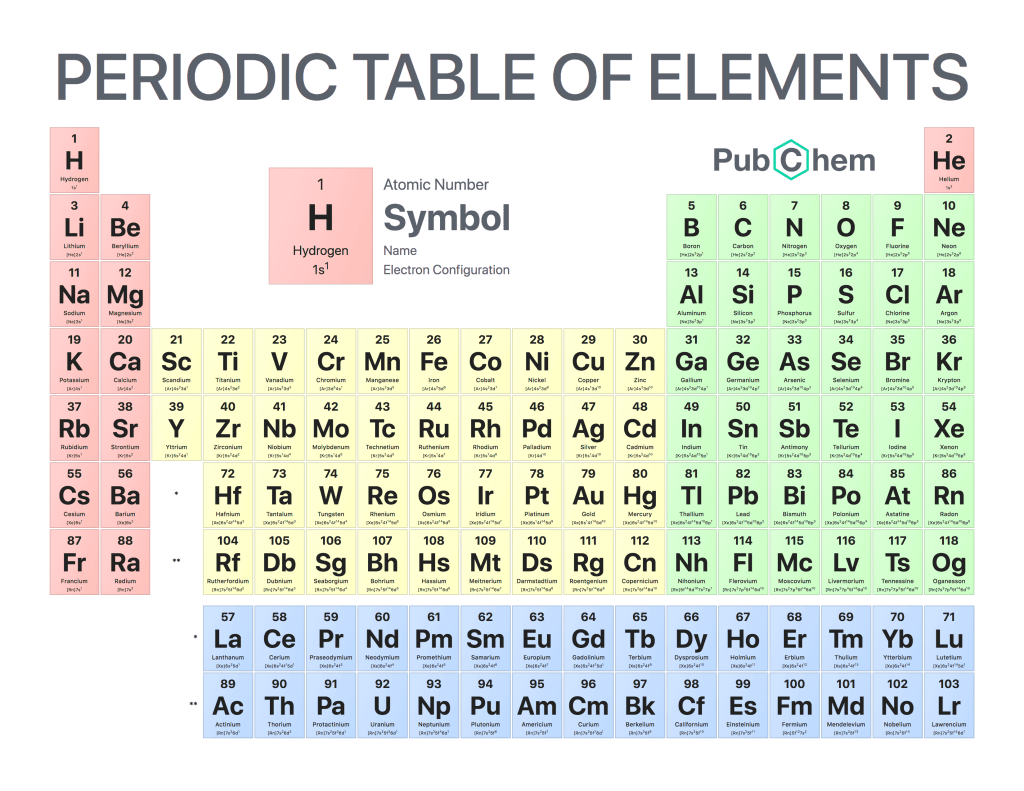
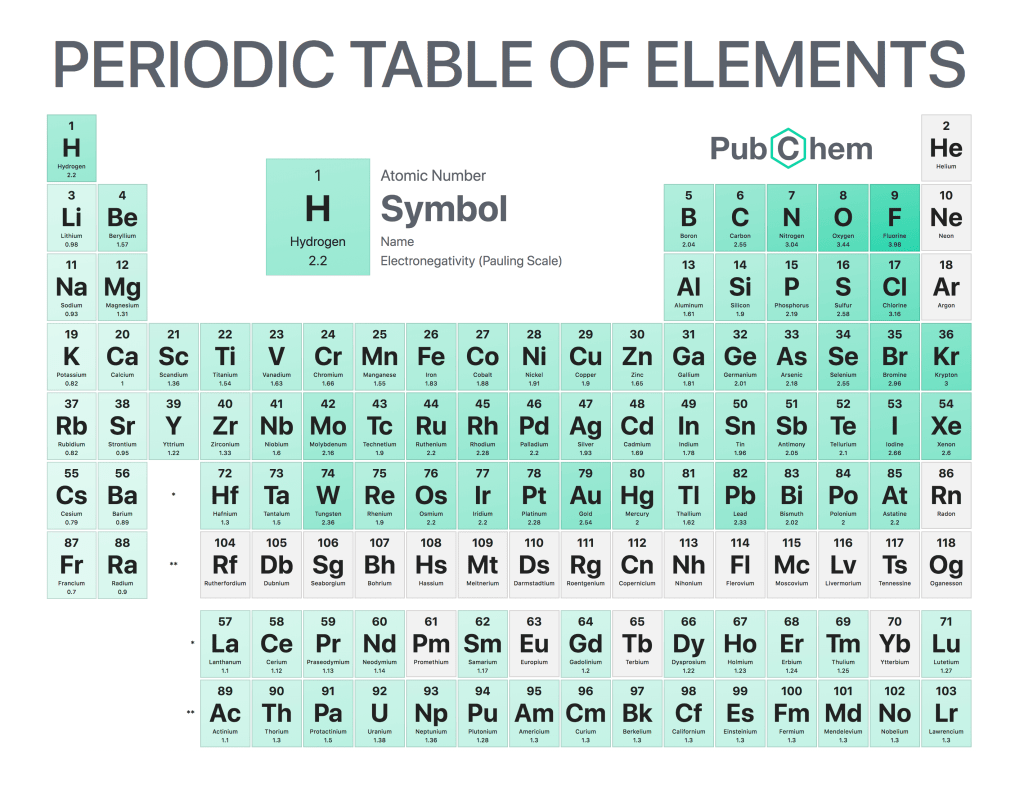
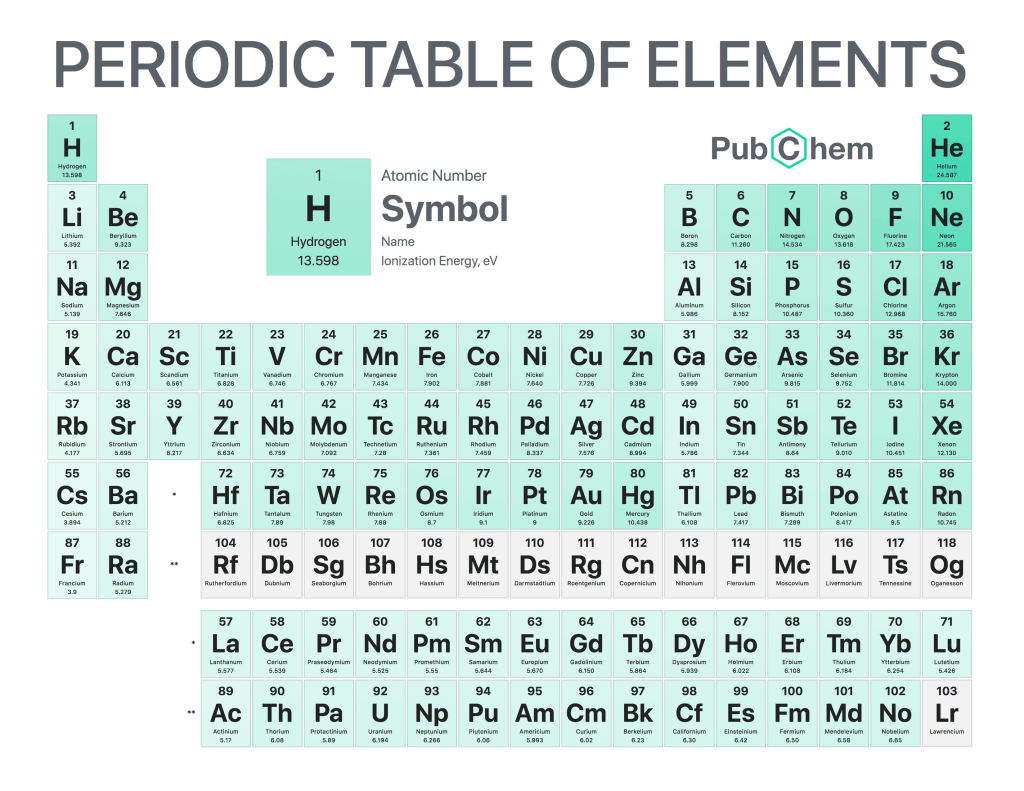
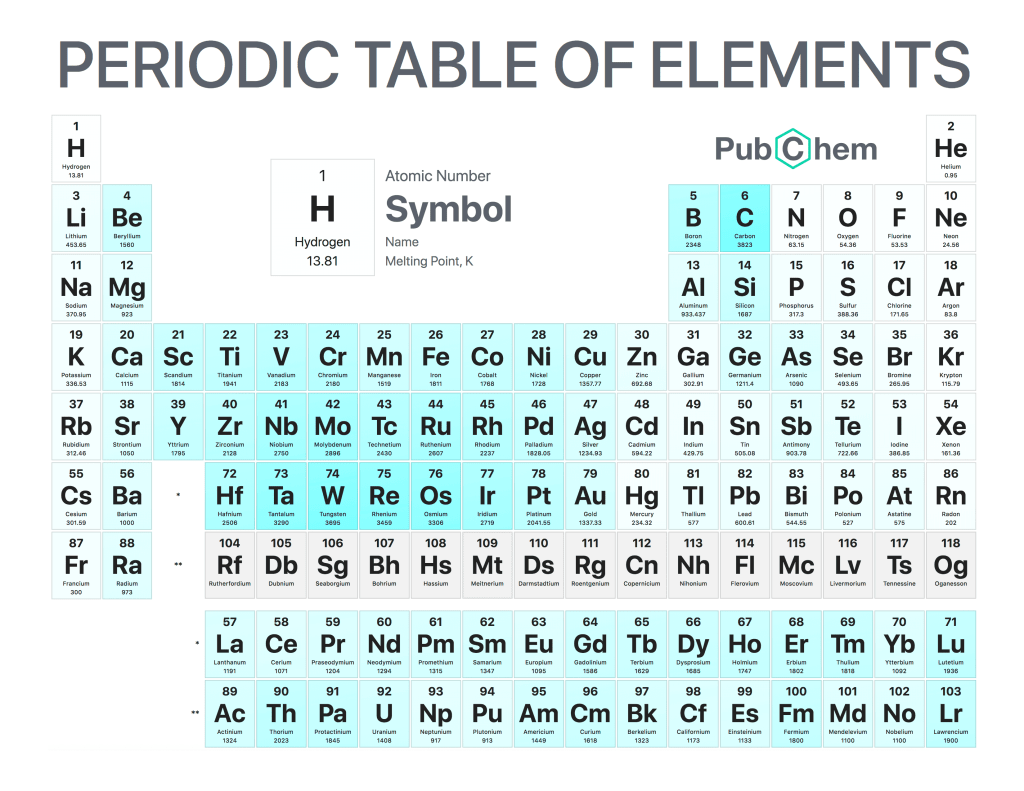
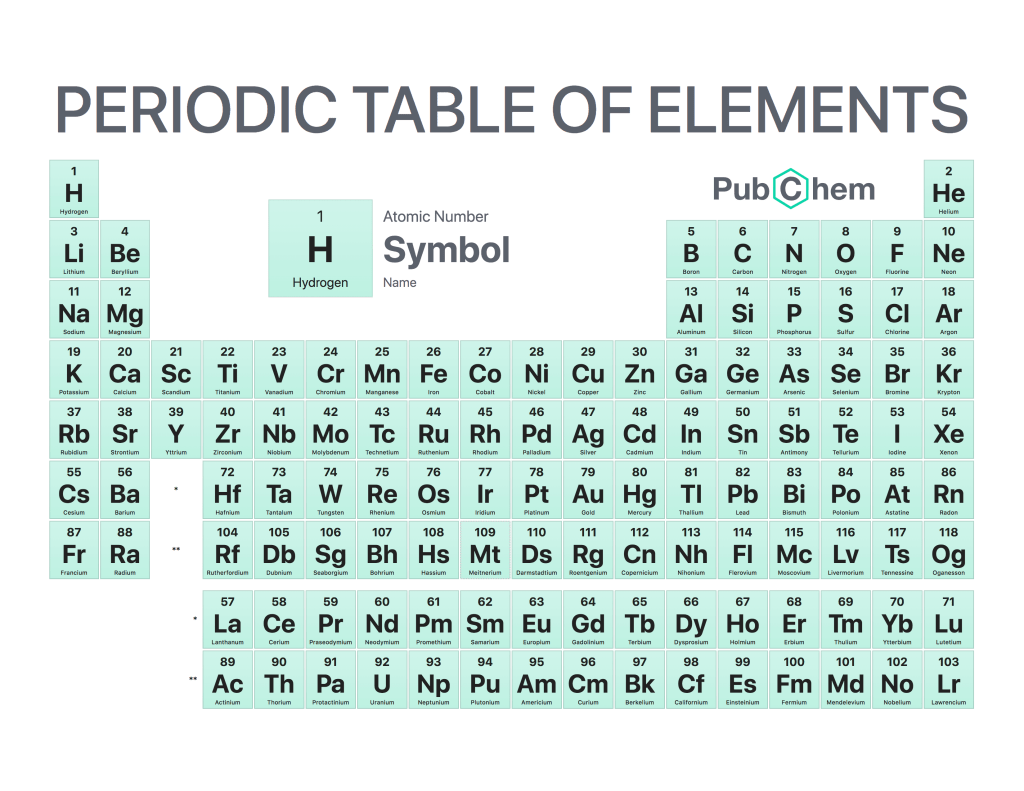
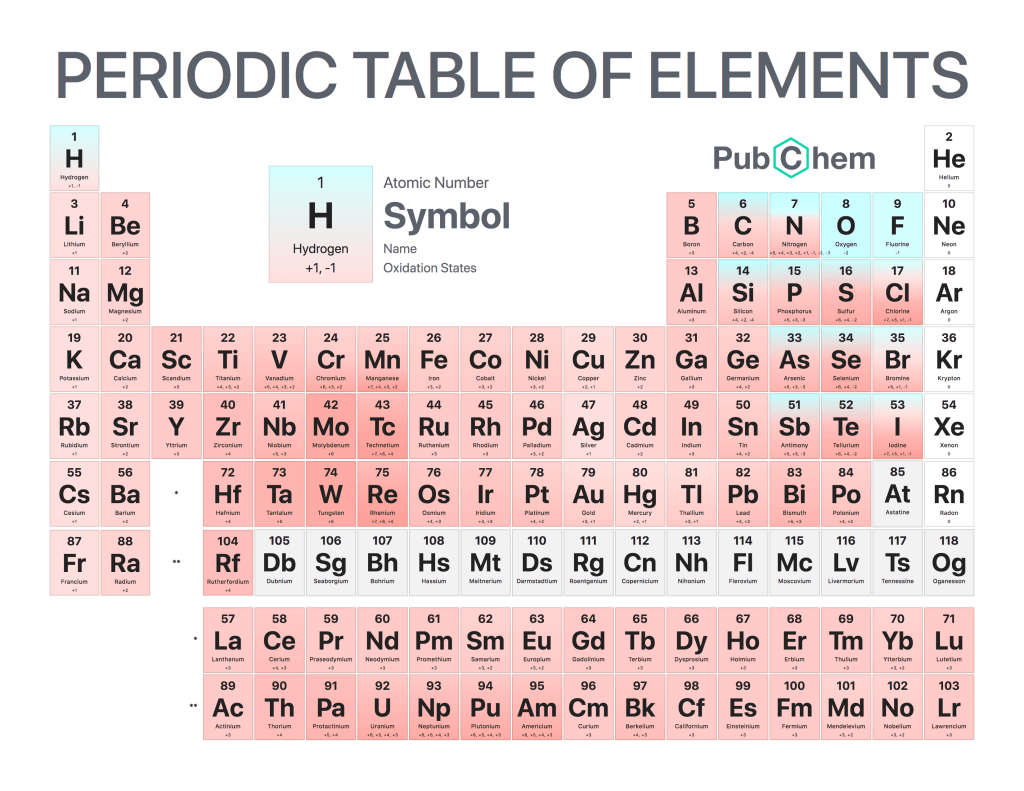
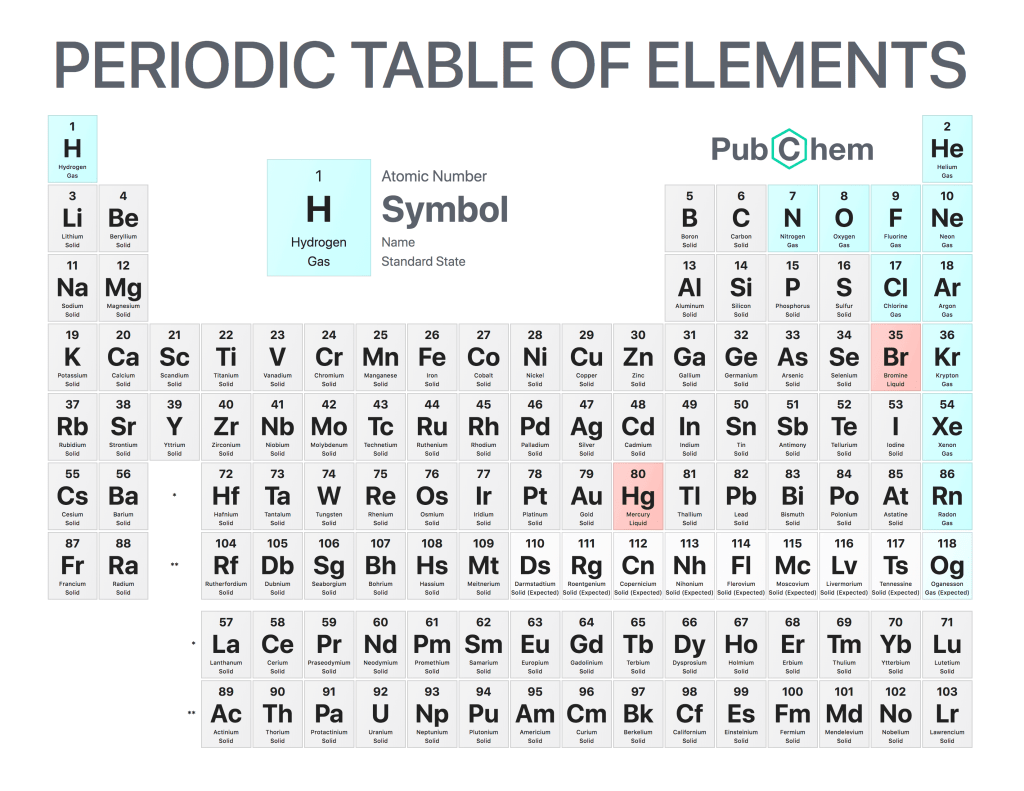
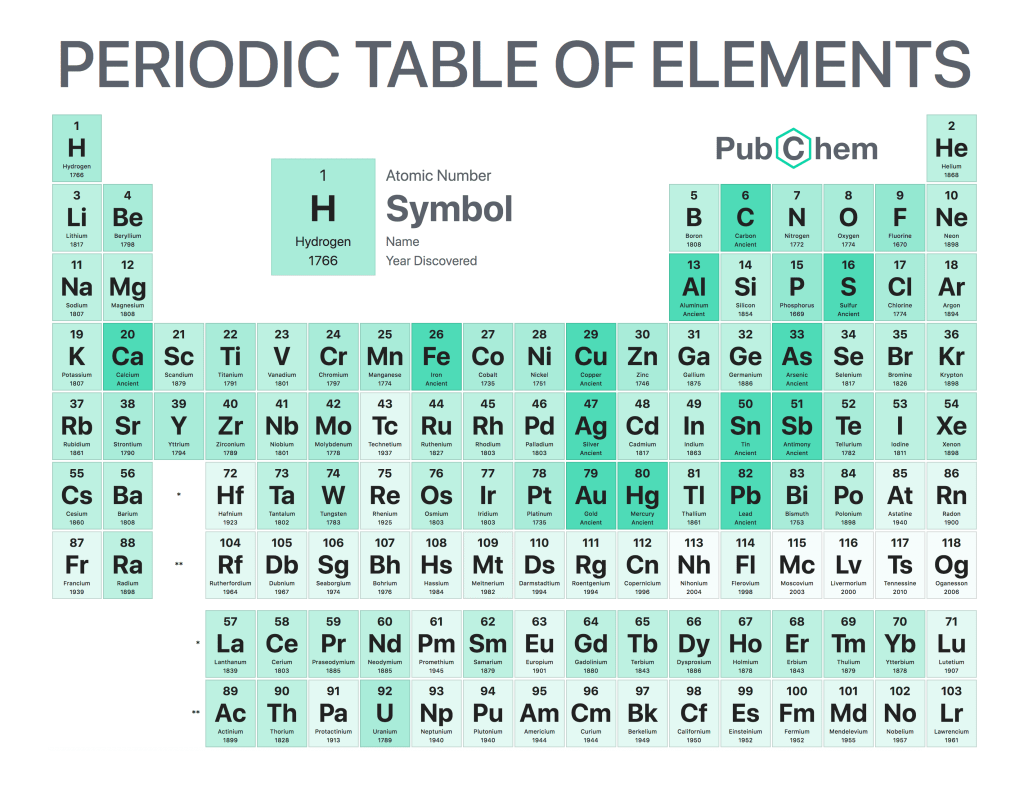
The periodic table of elements is a tabular arrangement of chemical elements, ordered by their atomic number, electron configurations, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups, contain elements with similar chemical behaviors. Underneath each element’s names are its atomic number—the number of protons in its nucleus—and its symbol.
The first element is hydrogen, which has an atomic number of 1. The second element is helium, which has an atomic number of 2. The third element is lithium, which has an atomic number of 3, and so on.
The periodic table can be divided into four sections: metals, metalloids, nonmetals, and noble gases. Metals are found on the left side of the table (except for hydrogen), while nonmetals are found on the right side (except for helium). Metalloids occupy a space between metals and nonmetals and have properties that are intermediate between those two groups. Noble gases are located at the far right side of the table (except for hydrogen) and do not react with other elements under normal conditions.
Elements in the same column have similar chemical properties because they have the same valence electrons—the electrons in their outermost energy level that determine how they interact with other atoms
Elements in the same column generally behave similarly because they have the same number of valence electrons—electrons in their outermost orbital that are available to form bonds with other atoms. For example, all noble gases except for helium (He) has eight valence electrons; as a result, they are all unreactive under standard conditions because they already have full valence shells.