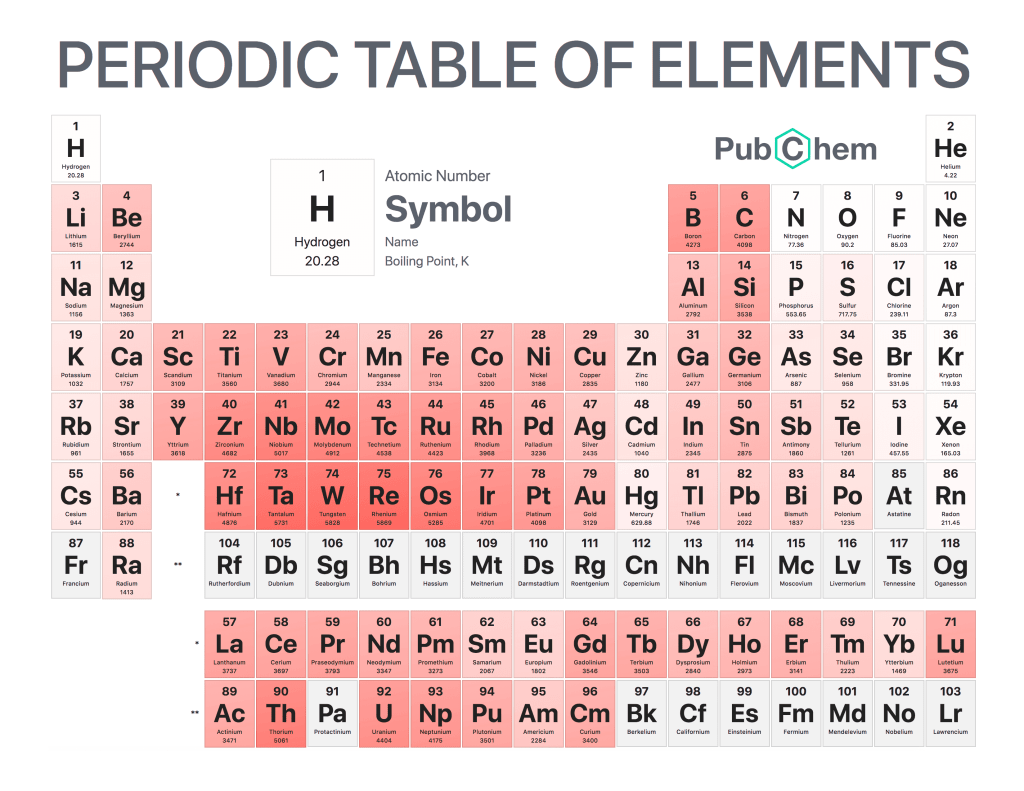
Boiling Point is a term used to describe the temperature at which a liquid changes from its liquid form into its gaseous state. This occurs when the atmospheric pressure on the surface of the liquid is equal to or greater than that of vapor pressure within it. The boiling point can vary depending on various factors, such as elevation and the type of substance being heated. Knowing boiling points for different substances can be useful in many applications, including cooking and laboratory experiments.
The boiling point for water is 212°F (100°C) under standard atmospheric conditions; however, this value varies with altitude due to differences in air pressure at higher elevations. For example, at sea level water boils at 212°F while it only boils around 180°F once you reach an altitude over 10 thousand feet above sea level! On top of this variation with elevation, there are also variations based on what other substances are present within a liquid mixture – these mixtures often have lower boiling points than pure liquids because they require less energy input before reaching their vaporization temperature threshold.
Knowing how each individual component affects boiling point allows us to better control our heating processes when attempting tasks like melting chocolate or creating concentrated solutions like sugar syrup! It’s important that we understand all variables involved so we don’t end up ruining our recipes by using too much heat or not enough heat – both scenarios could lead to disastrous results! Being aware of specific temperatures associated with certain materials will help ensure successful outcomes no matter what project you may be tackling next time around your kitchen countertop!
