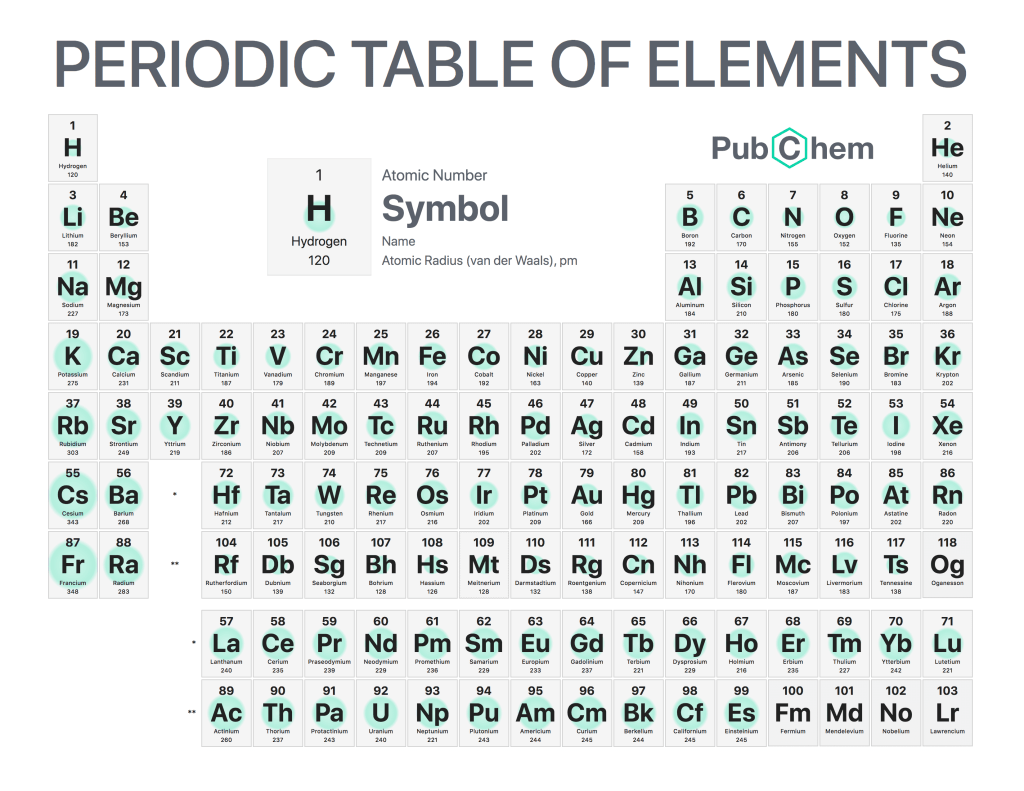
Atomic radius is a measure of the size of an atom, which is determined by the distance between its nucleus and outermost electron shells. The atomic radius can be calculated using various methods such as X-ray diffraction, crystallography, or spectroscopy. It has been found that atomic radius generally decreases across each period in the periodic table and increases down each group. This trend arises due to changes in nuclear charge within different elements; as protons are added to increase nuclear charge, electrons become more strongly attracted causing them to move closer towards the nucleus thus decreasing atomic radii across a period.
The influence of valence electrons on atomic radii can also be observed; when comparing two elements with a similar number of core electrons but differing numbers of valence electrons (elements from the same group) it has been noted that the element with a greater number of valance electron will have larger atoms due to increased shielding effect provided by additional outer shell resulting in reduced attraction between nuclei and inner shells hence increasing the overall size. For example, Chlorine (Cl) has 17 protons, 17 neutrons & 17 electrons whereas Argon (Ar) has 18 protons, 22 neutrons & 18 electrons; even though both have the same amount of core electrons Ar will still have a larger atomic radius than Cl because it possesses an additional valance electron which provides additional shielding effect to its outer shell thereby reducing the attraction between the nucleus and inner electrons shells leading to a larger atom size.
Finally, another factor influencing Atomic Radius is bond type i.e. ionic or covalent bonds. Generally speaking, ionic bonds tend to produce smaller atoms compared to covalent ones since they involve the complete transfer of one atom’s entire set electronegativity other thereby forming ions having very strong electrostatic forces binding them together tightly and reducing their overall sizes while on other hand covalently bonded molecules don’t form ions instead sharing pair/multiple pairs their own single orbital leading weaker bonding forces allowing for relatively large distances remain present between individual particles making up molecule thus producing comparatively bigger sized molecules than those formed via an ionic bonding process.
