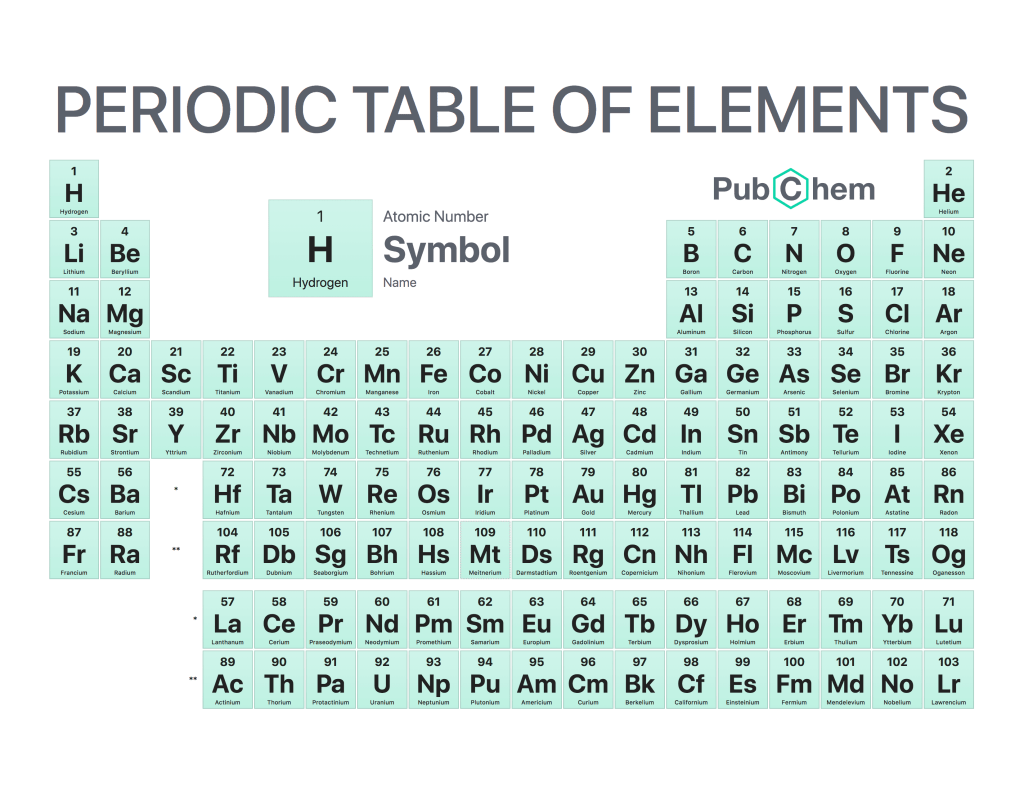
The atomic name is an important concept in the field of chemistry. It refers to a unique way of identifying each element on the periodic table by its chemical symbol, which consists of one or two letters. Atomic names are used as shorthand for elements and can be found in almost every scientific discipline.
Atomic names are based on Latin words that denote properties associated with each element, such as their mass number or atomic weight. For example, iron has an atomic name “Fe” which stands for Ferrum meaning “strong” because it is a strong metal with many uses throughout history including weapons and tools making up civilization’s infrastructure over time. Another example would be oxygen whose atom name comes from “oxys” meaning acid due to its acidic nature when combined with other elements like hydrogen creating water (H2O).
In addition to providing scientists and researchers quick reference points when discussing different elements, understanding how these symbols work together can help us gain insight into our world around us through a better understanding of what makes up the matter at the most basic level – atoms! By learning about how atoms combine together we can learn more about why certain materials react differently than others giving us valuable knowledge that helps shape our modern lives today!
