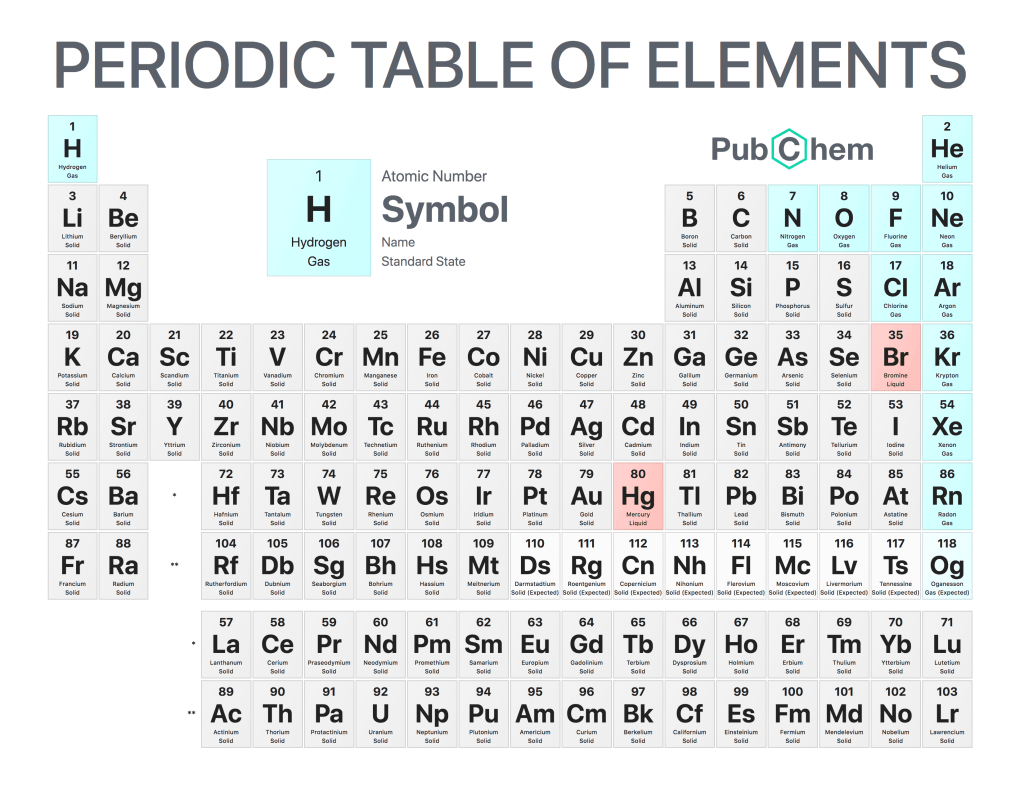
The Standard State Periodic Table is an essential tool for any chemistry student. It is a visual representation of the elements and their properties, allowing students to quickly recall information about each element, such as a state of gas, liquid, or solid state. The table was first developed by Russian chemist Dmitri Mendeleev in 1869 and has become the standard form used today.
The Standard State Periodic Table consists of 118 elements organized into seven rows or “periods” that are numbered from one to seven starting at the top left corner with hydrogen (H). Each row contains a specific number of elements that share similar chemical properties due to their atomic structure; this allows chemists to predict how different combinations will react when mixed together. In addition, each element also appears on its own block which includes details such as its atomic number, symbol, name, and relative mass among other important data points regarding its physical characteristics like melting point and boiling point, etc.
In conclusion, understanding the Standard State Periodic Table is an invaluable resource for anyone studying chemistry or related sciences as it provides quick access to key information about all known chemical compounds in existence today! This makes learning much easier since you can easily refer back when needed without having memorized all individual facts associated with every single element – so definitely be sure to make use of this great tool whenever possible!
