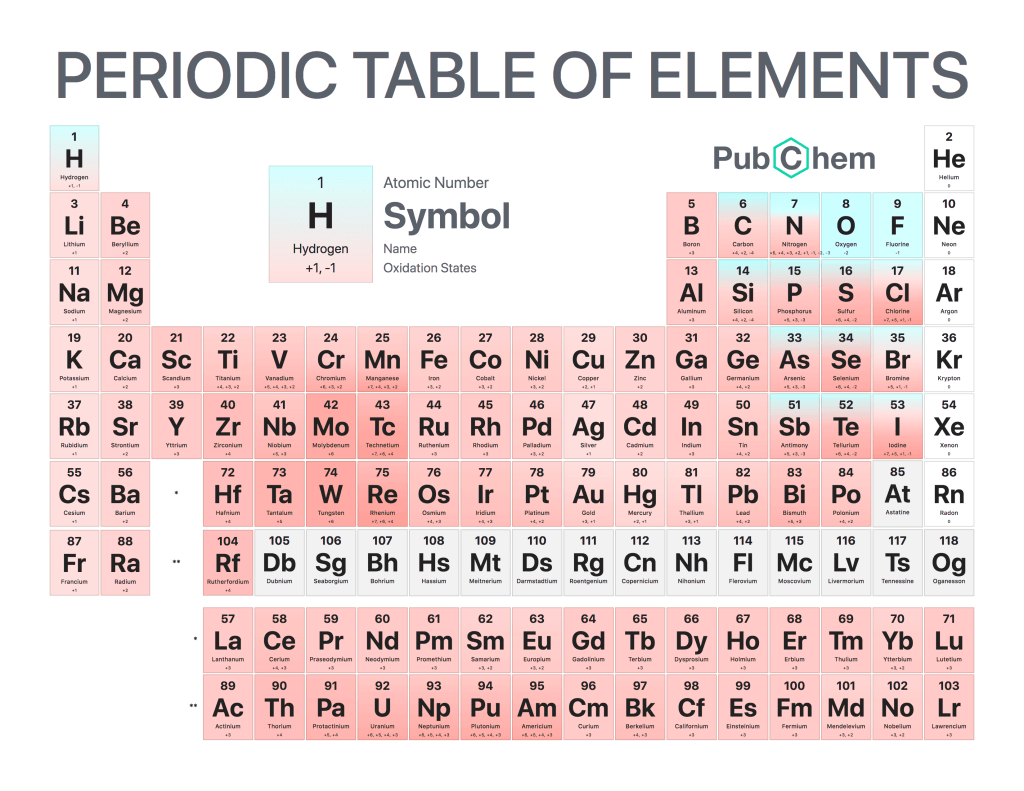
Oxidation states, also known as oxidation numbers, refer to the charge of an atom in a molecule or ion. They are useful for determining which element is oxidized and which element is reduced during a reaction. Oxidation states are important because they can help predict how molecules will react with each other and provide insight into their chemical properties.
The rules for assigning oxidation states vary depending on the type of bond involved but generally involve counting up electrons from different atoms in order to determine the overall charge of each one. For example, if two hydrogen atoms form a single covalent bond then their combined oxidation state would be zero since both have an electron count of one (H+ + H- = 0). On the other hand, if there were three oxygen atoms forming two double bonds then each oxygen’s individual oxidation state would be -2 since it has six electrons (O= + O= -6).
In some cases, elements may have more than one possible oxidation state due to complex bonding structures or resonance effects such as carbon dioxide where both oxygens can exist at either +4 or -2 depending on their environment. Therefore understanding these various rules and principles allows chemists to accurately assign proper charges when analyzing reactions so that they can better understand what happens between them chemically speaking.
