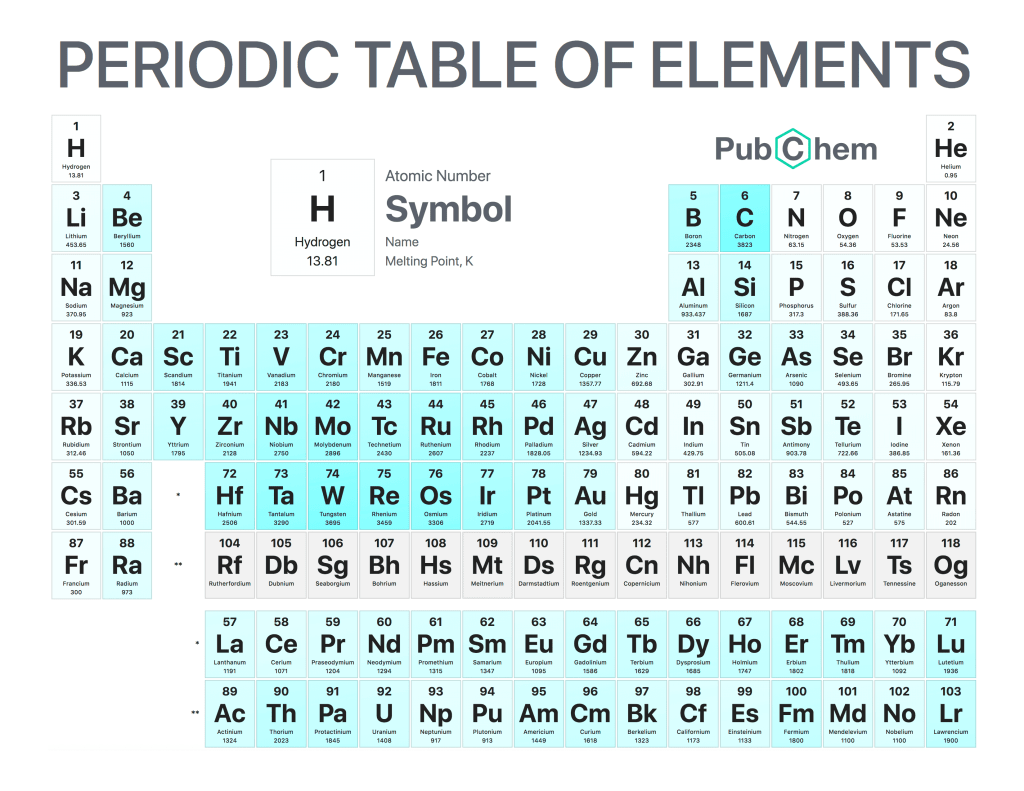
The melting point of a substance is the temperature at which it changes from a solid to a liquid. It is an important physical property used to identify and characterize substances, as well as being used in industry for quality control processes. The melting point can also be referred to as the freezing point, although this term is more often applied when discussing liquids that become solids upon cooling.
The melting points of different materials vary widely depending on their chemical composition and structure. For example, metals like iron have relatively high melting points (around 1535°C) while waxes like paraffin melt at much lower temperatures (around 60-65°C). Additionally, some materials may show polymorphism – meaning they exist in multiple forms with different structures that each have their own unique set of properties including distinct melting points or ranges of temperatures over which they will transition between solid and liquid phases.
In industrial applications such as manufacturing or food processing plants where accurate monitoring and control are crucial for product quality assurance purposes, precise measurements of the material’s exact boiling/melting/freezing points are essential. Many instruments exist specifically designed for this purpose such measuring devices include thermometers, differential scanning calorimeters, refractometers, etc. By using these tools one can ensure that products meet certain standards before entering the marketplace. Furthermore, proper knowledge about specific material’s thermal behavior will help manufacturers determine optimal conditions during the production process thereby reducing costs associated with energy consumption & wastage caused by incorrect heating methods.
Overall understanding how various substances respond under heat allows us not only to classify them but also optimize our use of resources efficiently & safely ensuring the highest possible level of safety standards for both workers & consumers alike!
