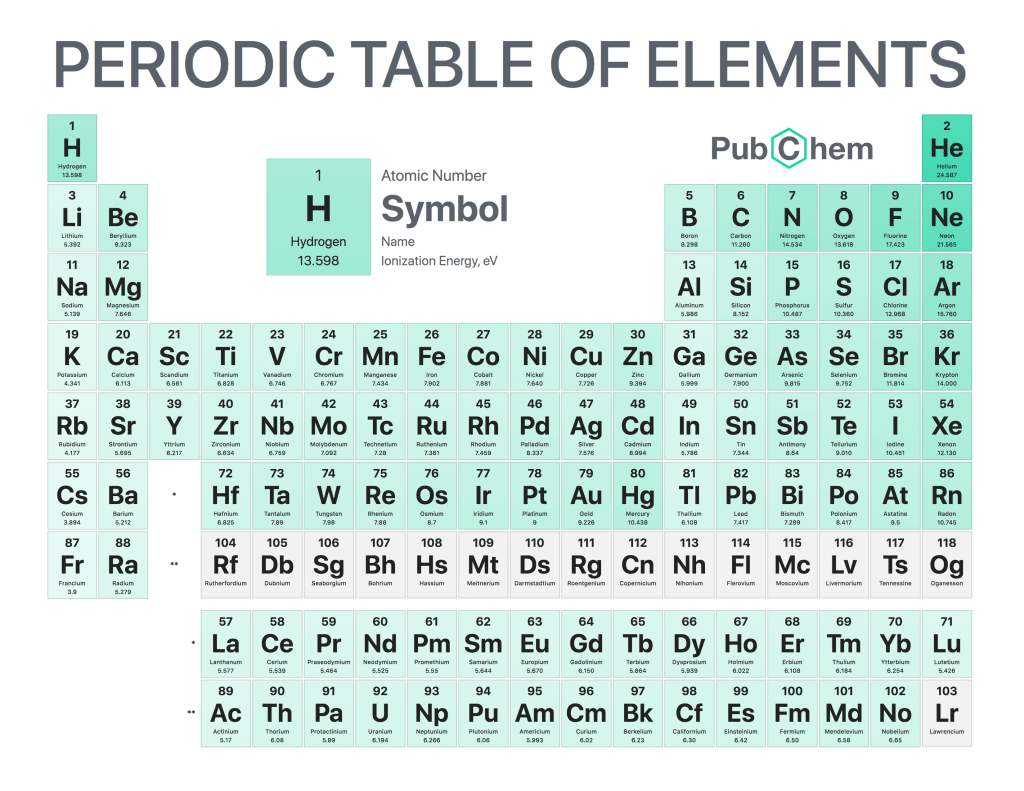
Ionization energy, also known as ionization potential, is the minimum amount of energy required to remove an electron from a gaseous atom or molecule. It can be thought of as the opposite of electronegativity – while electronegativity measures how strongly atoms attract electrons, ionization energy measures how strongly they hold onto them. This property has important implications in many areas such as chemistry and physics.
The first principle governing ionization energies states that it increases across a period on the periodic table due to increasing nuclear charge; this means that elements further down in a period have higher values than those above them because their nuclei are more positively charged and thus exert a greater attraction on their valence electrons (the ones closest to being removed). The second principle states that it decreases down groups because there is an increased shielding effect from inner shells which reduces the force with which outermost electrons are held by nuclei; this makes elements lower down in groups easier to remove an electron from compared with those at higher levels up within its group.
Finally, one should note that trends for ions differ somewhat depending on whether they are monatomic or polyatomic species – specifically for monatomic species (such as Na+), successive removal of additional electrons becomes increasingly harder whereas for polyatomic molecules like CO2 successive removal gets progressively easier after each loss since repulsion between remaining negative charges helps reduce overall binding strength per unit charge. These nuances must be taken into account when considering applications related to ionic interactions such as electrostatic forces between particles/molecules etc., so understanding these principles will always remain a key part of any scientific investigation involving chemical reactions!
