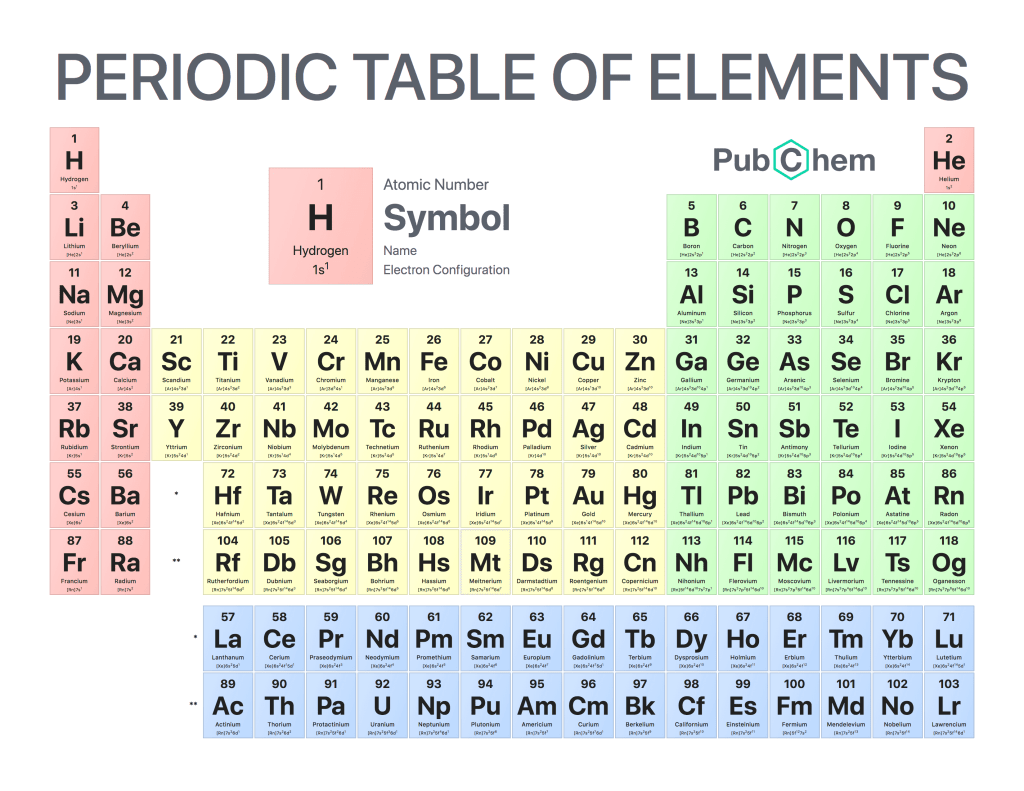
Electron configuration is a way of describing the arrangement of electrons in an atom. It helps to explain how atoms interact with each other and form chemical bonds, which are essential for life as we know it. Electron configuration can be written using different notation systems, but all describe the same underlying principle: that electrons occupy specific energy levels around an atom’s nucleus.
The most common notation system used to write electron configurations is called orbital diagrams or box diagrams. This method uses boxes arranged in concentric circles around a central nucleus symbol to represent each energy level and its associated sublevels within that level. Each box contains one or more arrows pointing outward from the center representing individual electrons occupying those orbitals; their direction indicates whether they have spin-up (↑) or spin-down (↓) orientation relative to their respective orbitals’ axes of rotation. The number of arrows per box corresponds with how many valence electrons there are at that energy level; two arrows indicate two valence electrons, three indicate three valence electrons, etc., up until eight total for any given orbital diagram—the maximum allowed by quantum mechanics’ Pauli exclusion principle before another shell must be added on top of additional space needs filling out further away from the nucleus symbol itself.
Finally, when writing electron configurations correctly according to this system you should always list them starting at the lowest available energies first and then moving upward towards higher ones lastly—this ensures accuracy between different notations being used across various scientific disciplines like chemistry & physics alike due its consistent nature throughout all applications regardless of what type of element/atom being studied! Doing so will help us better understand fundamental interactions occurring within our universe thus allowing us to gain greater insight into why certain phenomena occur such as why metals conduct electricity better than nonmetallic substances do among countless other topics related thereto too!
