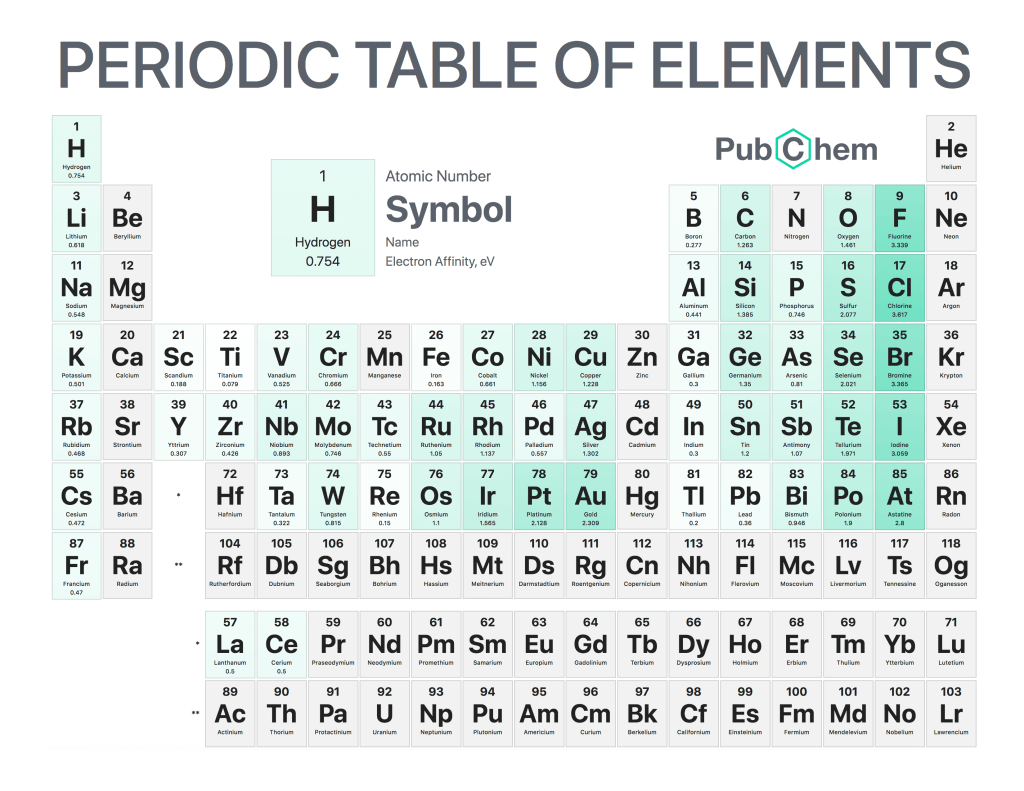
Electron affinity is the energy released when an atom gains an extra electron. It is a measure of how much energy must be used to add one more electron to a neutral atom, and it can vary significantly from element to element. Electron affinity plays an important role in chemistry because it helps explain why certain elements form chemical bonds with each other and why others do not. It also explains the behavior of electrons in different materials, such as metals or semiconductors.
The magnitude of electron affinity depends on several factors, including atomic structure and nuclear charge. Atoms that have larger nuclear charges usually have higher affinities for electrons than atoms with smaller charges because they can hold onto them more easily due to their greater attraction force between protons and electrons within the nucleus itself.. Additionally, atoms that contain fewer inner-shell (core) electrons tend to have higher affinities than those with many core electrons since there are fewer repulsive forces pushing away any additional incoming ones into outer shells. For example, Oxygen has six valence (outer shell ) electrons but only two core ones so its EA value is high compared to Fluorine which has seven valences but four cores resulting lower EA value.
In conclusion, Electron Affinity plays a key role in understanding chemical bonding between elements by helping predict whether or not two given species will interact favorably enough for bond formation. Its magnitude varies depending on various properties like the number of core/valance electrons &nuclear charge which determines the strength/weakness of attractive force towards incoming electrons thus making predictions easier while dealing with complex molecules.
