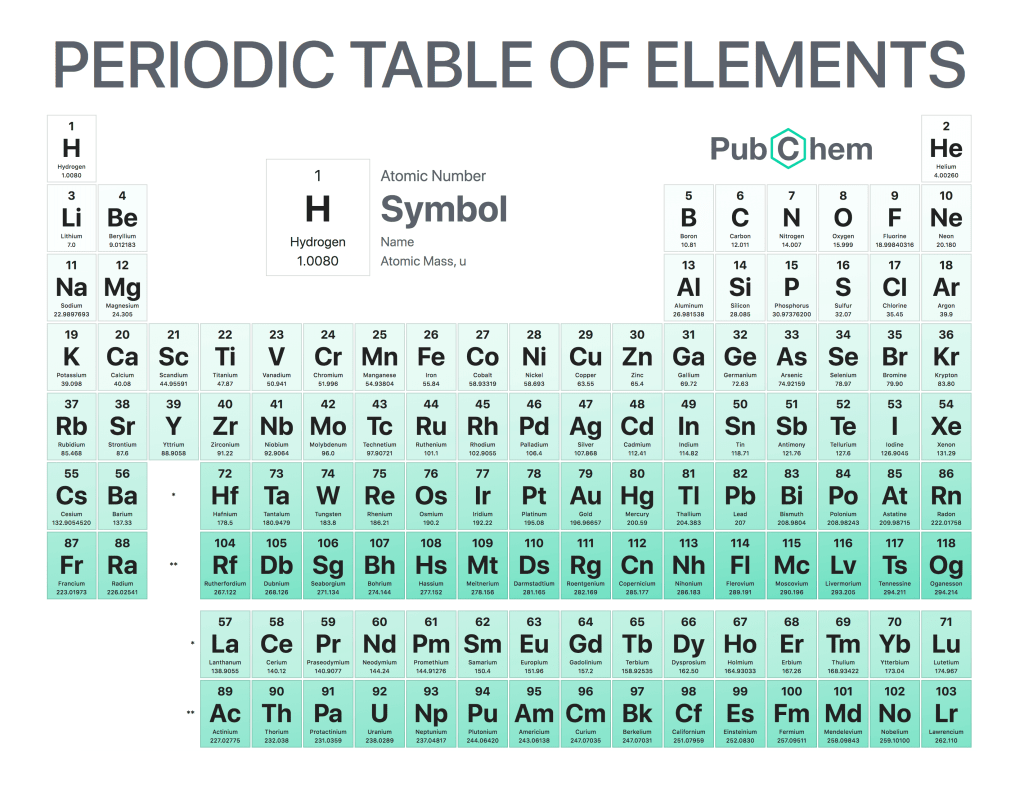
Atomic Mass is an important concept in chemistry and physics. It refers to the amount of matter that is contained within a single atom or molecule. Atomic mass can be expressed in either gram per mole (g/mol) or atomic mass units (AMU). The unit of measure for atomic mass was originally established by chemist John Dalton, who defined it as 1/12th the weight of one atom of carbon-12. This definition has since been refined and updated to take into account other elements and isotopes, but still remains at 1/12th the weight of carbon-12 as its base measurement unit.
The importance of understanding atomic mass lies primarily in its ability to provide insight into how atoms interact with each other on both a macroscopic level such as chemical reactions and a microscopic level such as nuclear fusion processes. By comparing different elements’ masses relative to each other we can understand why they react differently under certain conditions; this knowledge helps scientists create new materials or products from existing ones through careful manipulation at an elemental level while avoiding unwanted side effects like explosions!
In conclusion, understanding atomic mass provides us with valuable information about how different substances will behave when combined together which allows us to make informed decisions regarding their use for various applications ranging from medicine production all the way up space exploration missions! Knowing what kind of reaction you’ll get out of any given combination gives chemists more control over their experiments so they can achieve desired results without running too many risks along the way – making it an essential tool in modern-day science research fields!
